Common terminology and fundamental ideas
-
What is Series
-
What is Parallel
-
Cell Specifications (size, discharge, charge, chemistry)
-
Nominal voltage and charge/discharge practice
So, here you are, preparing to build yourself a nice battery. Although you have a long journey ahead of you, it’s important to know the terminology and basic ideas of battery building before you proceed.
Firstly, it’s important to know that every battery has a positive (+) and a negative (-) side. No matter the form factor of the battery, these must be present and are our way of interacting with the battery.
For our purposes, let’s look at the popular Samsung 30q battery, which is in a 18650 form factor. As pictured on the right, the first cell (left) displays the positive (or “anode”), whereas the right cell displays the negative (or “cathode”).
This leads us to the next fundamental idea- form factor. As aforementioned, the 30q is in the 18650 form factor, but what does this mean? Yes, there is a method to this madness. The first two numbers represent the width of the cell, in this case, its 18mm (yes obviously its in metric, its superior). The next two numbers represent the length of the cell, in this case, 65mm.

Now obviously other form factors exist such as 21700 cells (21mm wide, 70.0mm long), which follows the same naming scheme. This rule applies only to cylindrical cells, and makes it very easy to understand their sizes.
Now, onto terminology. Building a battery consists of connecting individual cells in series or parallel to construct a bigger battery. But what does that even mean? Well:
- Connecting cells in series means connecting them positive to negative
- Connecting cells in parallel means connecting them positive to positive and negative to negative
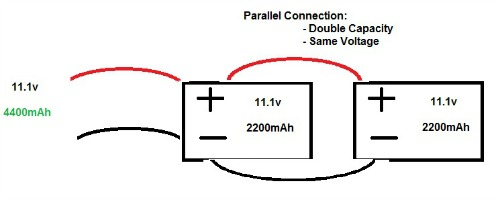
As can be seen from the above diagrams, connecting in series or parallel has a different effect on the characteristic of the battery. Sometimes, you may see shorthand used, such as 12s4p, which denotes 12 cells in series, and 4 cells in parallel. When connecting cells to build a battery, it is important to remember the basic rules of circuitry:
- Don’t cross (connect) the positive and negative of a cell, or of the battery pack.
- In order to connect cells in series, they must be similar in capacity, or be of identical parallel count.
- Know your system’s capabilities in order to not exceed the specification of any one given part.
Now it is the third rule that leads us to our next point. Irregardless of the form factor, each cell has its own specifications and capabilities, and it is important that these are understood to ensure a safe battery.
For example, the aforementioned 30q is rated for 3000mAh (milli-amp hours) and at 15 Amps maximum discharge. If these specifications are exceeded, you may cause damage to the cell (possibly irreparable), or even cause a complete failure. Now, the details of this are semi-complicated, so let’s look at each of the aspects individually.
-
Capacity- The capacity of a cell is normally rated in mAh. Like any scientific unit, if the capacity is large enough, it may also be written as Ah with any prefix. Now many of us are used to seeing capacity rated on a 1-100 percentage scale. However, that’s not actually how it works behind-the-scenes. Continuing to use the 30q’s as an example, the maximum voltage per cell is 4.2v. This is equivalent to 100% state of charge. 2.8 - 3v is the minimum cell voltage, this is equivalent to 0% state of charge. Anything below this is dangerous to you and the cell. Cautionary tales are told of those who have successfully revived cells from these lower voltages, but as a beginner, I would urge you to stay away from taking such risks. The range between 4.2v and 2.8v is about 3000 mAh for the 30q. The higher the mAh rating, the more capacity you have.
-
Discharge- The Amp rating (or C rating for LiPo’s) details how many amps can be drawn from a cell. First, it’s important to understand the relationship between current and heat. The higher the current, the higher the heat. When discharging cells closer to their maximum rated discharge, they are likely to begin to heat up. The increased heat will limit current draw, and if the temperature becomes excessive, can become problematic. Furthermore, heat energy is wasted energy, so keeping your packs cool is important for efficiency and safety.
Here is where the line blurs a little. With certain cells, you can safely discharge them at higher amps than what they are rated for, given that they stay cool. The 30q is one of these cells. Although rated for a max of 15A discharge per cell, many have found that they are safe for a 20A discharge. This is not a behavior present in all cells, and it is at your own risk, so please conduct appropriate research prior to attempting to push your cells over what they are rated.
A trusted source for cell specs is @Battery_Mooch, who tests a variety of cells himself, and gives realistic ratings for cells. Sometimes the manufacturer’s specification sheet is inaccurate (over or under rated), when choosing cells conduct thorough research as to what they are capable of, and if they are suitable for your intended use.





